EXPERIMENTAL INVESTIGATION OF AMINE-BASED GRAPHENE NANOSUSPENSION FOR CO2 ABSORPTION
Main Article Content
Abstract
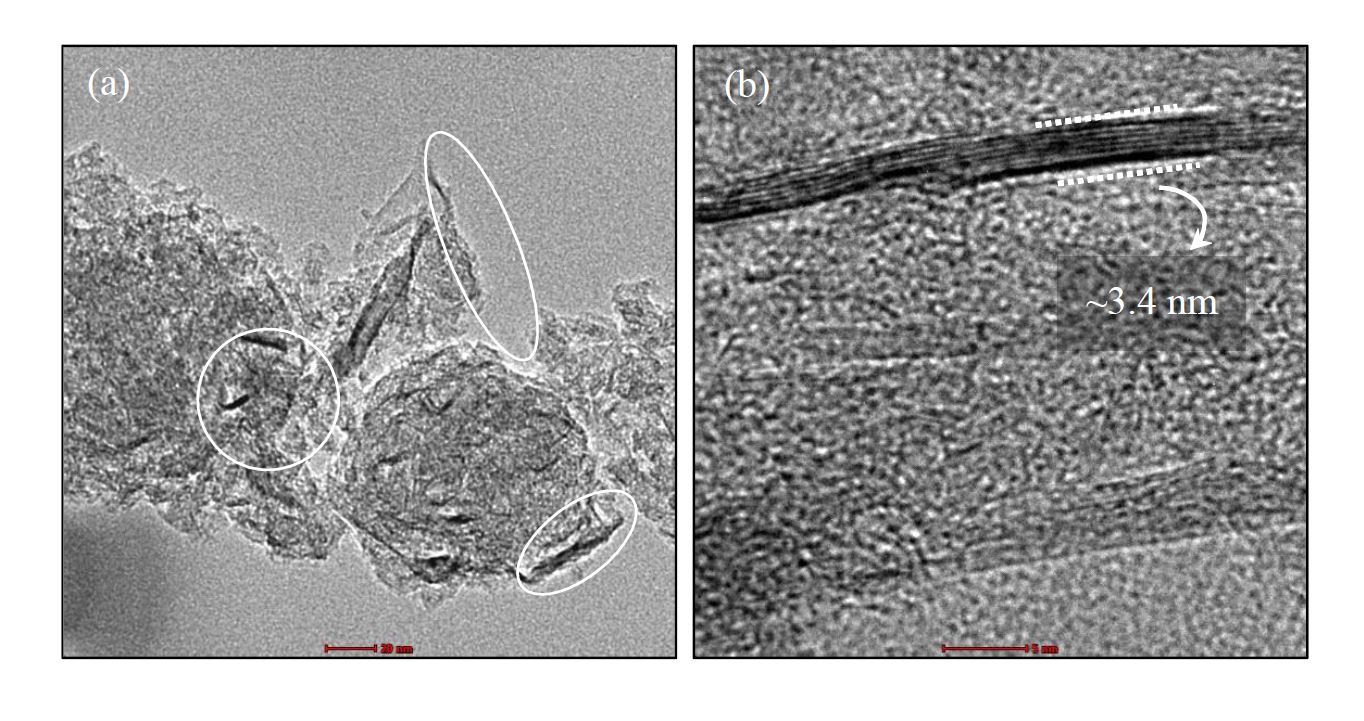
Absorption is the most widely used carbon dioxide (CO2) removal technology. The CO2 absorption performance of monoethanolamine (MEA), the most commonly used CO2 absorbent, can be improved by suspending nanoparticles. This work examined the performance of graphene nanoplatelets (GNPs) as additives to enhance CO2 absorption in MEA. The GNPs were characterized by HRTEM, FTIR, and XRD. The study examined the influence of GNP concentrations on CO2 absorption at room temperature. The images from HRTEM confirmed that the implemented graphene consists of several layers of graphene sheets. Increasing the loading of particles increased the solubility of CO2 until the optimum concentration was reached. From this work, it is evident that incorporating GNPs into MEA enhances the CO2 absorption performance of MEA. Thus, the addition of nanoparticles to the absorbent can enhance its CO2 absorptivity.
Downloads
Article Details
Transfer of Copyrights
- In the event of publication of the manuscript entitled [INSERT MANUSCRIPT TITLE AND REF NO.] in the Malaysian Journal of Science, I hereby transfer copyrights of the manuscript title, abstract and contents to the Malaysian Journal of Science and the Faculty of Science, University of Malaya (as the publisher) for the full legal term of copyright and any renewals thereof throughout the world in any format, and any media for communication.
Conditions of Publication
- I hereby state that this manuscript to be published is an original work, unpublished in any form prior and I have obtained the necessary permission for the reproduction (or am the owner) of any images, illustrations, tables, charts, figures, maps, photographs and other visual materials of whom the copyrights is owned by a third party.
- This manuscript contains no statements that are contradictory to the relevant local and international laws or that infringes on the rights of others.
- I agree to indemnify the Malaysian Journal of Science and the Faculty of Science, University of Malaya (as the publisher) in the event of any claims that arise in regards to the above conditions and assume full liability on the published manuscript.
Reviewer’s Responsibilities
- Reviewers must treat the manuscripts received for reviewing process as confidential. It must not be shown or discussed with others without the authorization from the editor of MJS.
- Reviewers assigned must not have conflicts of interest with respect to the original work, the authors of the article or the research funding.
- Reviewers should judge or evaluate the manuscripts objective as possible. The feedback from the reviewers should be express clearly with supporting arguments.
- If the assigned reviewer considers themselves not able to complete the review of the manuscript, they must communicate with the editor, so that the manuscript could be sent to another suitable reviewer.
Copyright: Rights of the Author(s)
- Effective 2007, it will become the policy of the Malaysian Journal of Science (published by the Faculty of Science, University of Malaya) to obtain copyrights of all manuscripts published. This is to facilitate:
- Protection against copyright infringement of the manuscript through copyright breaches or piracy.
- Timely handling of reproduction requests from authorized third parties that are addressed directly to the Faculty of Science, University of Malaya.
- As the author, you may publish the fore-mentioned manuscript, whole or any part thereof, provided acknowledgement regarding copyright notice and reference to first publication in the Malaysian Journal of Science and Faculty of Science, University of Malaya (as the publishers) are given. You may produce copies of your manuscript, whole or any part thereof, for teaching purposes or to be provided, on individual basis, to fellow researchers.
- You may include the fore-mentioned manuscript, whole or any part thereof, electronically on a secure network at your affiliated institution, provided acknowledgement regarding copyright notice and reference to first publication in the Malaysian Journal of Science and Faculty of Science, University of Malaya (as the publishers) are given.
- You may include the fore-mentioned manuscript, whole or any part thereof, on the World Wide Web, provided acknowledgement regarding copyright notice and reference to first publication in the Malaysian Journal of Science and Faculty of Science, University of Malaya (as the publishers) are given.
- In the event that your manuscript, whole or any part thereof, has been requested to be reproduced, for any purpose or in any form approved by the Malaysian Journal of Science and Faculty of Science, University of Malaya (as the publishers), you will be informed. It is requested that any changes to your contact details (especially e-mail addresses) are made known.
Copyright: Role and responsibility of the Author(s)
- In the event of the manuscript to be published in the Malaysian Journal of Science contains materials copyrighted to others prior, it is the responsibility of current author(s) to obtain written permission from the copyright owner or owners.
- This written permission should be submitted with the proof-copy of the manuscript to be published in the Malaysian Journal of Science
Licensing Policy
Malaysian Journal of Science is an open-access journal that follows the Creative Commons Attribution-Non-commercial 4.0 International License (CC BY-NC 4.0)
CC BY – NC 4.0: Under this licence, the reusers to distribute, remix, alter, and build upon the content in any media or format for non-commercial purposes only, as long as proper acknowledgement is given to the authors of the original work. Please take the time to read the whole licence agreement (https://creativecommons.org/licenses/by-nc/4.0/legalcode ).
References
Abdul Samat, N. F. N., Yusoff, R. B., Aroua, M. K., Ramalingam, A., & Kassim, M. A. (2019). Solubility of CO2 in aqueous 2‑amino‑1, 3‑propanediol (Serinol) at elevated pressures. Journal of Molecular Liquids, 277, 207-216. doi: https://doi.org/10.1016/j.molliq.2018.12.102
Farinre, O. Z., Alghamdi, H., Kelley, M. L., Biacchi, A. J., Albert, V., Davydov, . . . Misra, P. (2022). A Comprehensive Study on the Molecular Dynamics of Pristine and Functionalized Graphene Nanoplatelets.
Gomari, S., Esfandeh, M., & Ghasemi, I. (2017). All-solid-state flexible nanocomposite polymer electrolytes based on poly(ethylene oxide): Lithium perchlorate using functionalized graphene. Solid State Ionics, 303, 37-46. doi: https://doi.org/10.1016/j.ssi.2017.02.005
Hamidi, R., Farsi, M., & Eslamloueyan, R. (2018). CO2 solubility in aqueous mixture of MEA, MDEA and DAMP: Absorption capacity, rate and regeneration. Journal of Molecular Liquids, 265, 711-716. doi: https://doi.org/10.1016/j.molliq.2018.07.013
Jiang, J., Zhao, B., Zhuo, Y., & Wang, S. (2014). Experimental study of CO2 absorption in aqueous MEA and MDEA solutions enhanced by nanoparticles. International Journal of greenhouse gas control, 29, 135-141.
Kumar, J. S., Murmu, N. C., Samanta, P., Banerjee, A., Ganesh, R. S., Inokawa, H., & Kuila, T. (2018). Novel synthesis of a Cu2O–graphene nanoplatelet composite through a two-step electrodeposition method for selective detection of hydrogen peroxide. New Journal of Chemistry, 42(5), 3574-3581. doi: 10.1039/C7NJ04510G
Lai, Q., Toan, S., Assiri, M. A., Cheng, H., Russell, A. G., Adidharma, H., . . . Fan, M. (2018). Catalyst-TiO(OH)2 could drastically reduce the energy consumption of CO2 capture. Nature Communications, 9(1), 2672. doi: 10.1038/s41467-018-05145-0
Mohammadpoor, A., Mirzaei, M., Azimi, A., & ghomshe, m. t. (2018). The simultaneous effect of graphene oxide and sodium dodecyl sulphate nanoparticles on the kinetics of CO2 absorption in amine. Advances in environmental science and technology, 4, 163-174.
Mohammadpour, A., Mirzaei, M., & Azimi, A. (2019). Dimensionless numbers for solubility and mass transfer rate of CO2 absorption in MEA in presence of additives. Chemical Engineering Research and Design, 151, 207-213. doi: https://doi.org/10.1016/j.cherd.2019.06.026
Ochedi, F. O., Yu, J., Yu, H., Liu, Y., & Hussain, A. (2021). Carbon dioxide capture using liquid absorption methods: a review. Environmental Chemistry Letters, 19, 77-109.
Rahimi, K., Riahi, S., & Abbasi, M. (2020). Effect of host fluid and hydrophilicity of multi-walled carbon nanotubes on stability and CO2 absorption of amine-based and water-based nanofluids. Journal of Environmental Chemical Engineering, 8(1), 103580. doi: https://doi.org/10.1016/j.jece.2019.103580
Ramezani, R., Mazinani, S., & Di Felice, R. (2021). Density, Viscosity, pH, Heat of Absorption, and CO2 Loading Capacity of Methyldiethanolamine and Potassium Lysinate Blend Solutions. Journal of Chemical & Engineering Data, 66(4), 1611-1629.
Seo, S., Lages, B., & Kim, M. (2020). Catalytic CO2 absorption in an amine solvent using nickel nanoparticles for post-combustion carbon capture. Journal of CO2 Utilization, 36, 244-252.
Zhang, H., Wang, B., Xiong, M., Gao, C., Ren, H., & Ma, L. (2022). Process intensification in gas-liquid mass transfer by nanofluids: Mechanism and current status. Journal of Molecular Liquids, 346, 118268.